Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Diacétyl - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 431-03-8
-
EINECS number : 207-069-8
-
FEMA number : 2370
-
Density : 0,981
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : Yellow liquid
-
FLAVIS number : 07.052
-
JECFA number : 408
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 88°C
-
Detection Threshold : De l'ordre de 0,3 à 15 ppb (0,0000015%). Son seuil de reconnaissance est de 5 ppb environ.
-
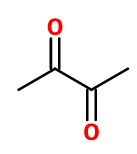
Molecular formula : C4H6O2
-
Log P : -0,47
-
Molecular Weight : 86,09 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 7°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to Acetoin, Diacetyl is much more used in perfumery. It also is more powerful.
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Diacetyl is not so often used in perfumery. Used in vanillic, caramel and fruity notes for a butter, natural and pastry effect.
Year of discovery :
Data not available.
Isomerism :
Diacetyl does not have any isomer used in perfumery.
Synthesis precursor :
Diacetyl is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Diacetyl is contained in many fruits and in butter, among others. It is extracted from certain fruits in its natural state.
Synthesis route :
Diacetyl is synthesized in several possible ways. The most common is the catalytic dehydrogenation (with copper and chromium) of 2,3-butanediol. Biochemical pathways are also widely used on an industrial scale.
Regulations & IFRA
This ingredient is not restricted

