Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Aldéhyde Cyclamen - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 103-95-7
-
EINECS number : 203-161-7
-
FEMA number : 2743
-
Density : 0,948
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 05.045
-
JECFA number : 1465
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 270°C
-
Detection Threshold : 2,51 ng/l air
-
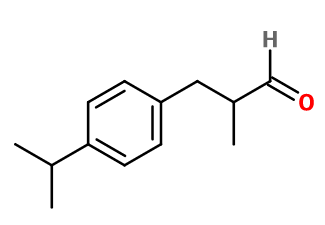
Molecular formula : C13H18O
-
Log P : 3,4
-
Molecular Weight : 190,28 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 109°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Cyclamen Aldehyde must not contain more than 1.5% cyclamen alcohol because it is an irritant.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Unstable in acidic products, except fabric conditioners, and in very alkaline products, such as bleach.
Uses in perfumery :
Cyclamen Aldehyde is used in marine and floral-green accords such as lily of the valley. Provides volume and an ozonic note.
Year of discovery :
Discovered in 1929. Patent N°1,844,013 published in October,18 1929 by Knorr.A and Weissenborn.A for Winthrop Chemical Company
Isomerism :
Cyclamen Aldehyde has an asymmetric carbon. It is the racemic mixture of the molecule that is used in perfumery. The ortho and meta isomers of Aldehyde Cyclamen are not used. Damascenone-Beta is a constitutional isomer of Cyclamen Aldehyde. Both have a very different smell: Damascenone-Beta is reminiscent of cooked apples.
Synthesis precursor :
Cyclamen Aldehyde Schiff base by reaction with Methyl Anthranilate has a smell of lily of the valley and orange blossom. Several acetals may also be formed by reaction with alcohols in an acid medium and have an olfactory interest.
Natural availability :
Cyclamen Aldehyde is not available in its natural state.
Synthesis route :
Cyclamen Aldehyde is synthesized by two different routes. The first is a condensation of two aldehydes: 4-isopropylbenzaldehyde and Acetaldehyde, with an excess of the first reagent and a slow addition of the second, to avoid self-condensation of the latter. This reaction is followed by a hydrogenation of the double bond formed during the process. The second synthetic route is a Friedel-Craft reaction between isopropylbenzene and methacrolein diacetate, followed by an acid hydrolysis of the intermediate product obtained.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION_SPECIFICATION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,11 % 0,14 % 0,038 % 0,95 % 0,45 % 0,076 % 0,076 % 0,025 % 0,076 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,076 % 0,076 % 0,025 % 0,23 % 0,23 % 0,72 % 0,025 % 0,025 % 16 %
Comments :
Cyclamen aldehyde should not contain more than 1.5% of Cyclamen alcohol. Cyclamen aldehyde has been found in natural extracts but only at trace levels.

