Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Citronellal - 30gr | - | - | - | - | - | - | more | - | |
|
|
Citronellal Natural 85%+ | CT-101 | Natural | 85 | Cymbopogon winterianus jowitt | Citronella Oil | Indonesia | more | 800 Kgs | |
|
|
Citronellal Natural 95%+ | CT-103 | Natural | 95 | Cymbopogon winterianus jowitt | Citronella Oil | Indonesia | more | 720 Kgs | |
|
|
Citronellal Natural 90%+ | CT-102 | Natural | 90 | Cymbopogon winterianus jowitt | Citronella Oil | Indonesia | more | 800 Kgs | |
|
|
Citronellal | 30035052 | Molecule | - | - | - | - |





|
more | - |
|
|
Citronellal BMBcert™ | 30786642 | Molecule | - | - | - | - |





|
more | - |
General Presentation
-
CAS N° : : 106-23-0
-
EINECS number : 203-376-6
-
FEMA number : 2307
-
Density : 0,857
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head/Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 05.021
-
JECFA number : 1220
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 207°C
-
Detection Threshold : Entre 31 et 100 ppb (0,00001%) selon les personnes
-
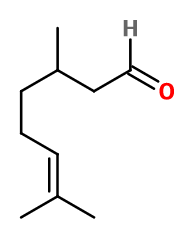
Molecular formula : C10H18O
-
Log P : 3,62
-
Molecular Weight : 154,25 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 86°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Citronellal is one of the 26 allergens in perfumery.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Terpenes tend to polymerize by oxydation.
Uses in perfumery :
Citronellal is used in lemon and lemongrass reproductions and for a citral and fresh effect in citrus notes. Can be used in rosy notes.
Year of discovery :
Discovered in 1889.
Isomerism :
Citronellal has an asymmetric carbon that gives rise to two enantiomers. Both isomers have a rather similar smell, although they are not necessarily present in the same plants. Usually, it is the racemic mixture of the two isomers that is used in perfumery. Eucalyptol, Geraniol and Linalool are examples of constitutional isomers of Citronellal. Their smell is however very different, as it is more floral and camphorated for Eucalyptol.
Synthesis precursor :
Citronellal is a precursor to the synthesis of many other perfume compounds. Depending on the catalyst, its hydrogenation enables the obtention of Citronellol, dihydrocitronellal or dihydrocitronellol. An acid hydrolysis allows to synthesize Hydroxycitronellal. Cyclizing the molecule by acid catalysis also allows to obtain Pulegol, a precursor for the synthesis of L-Menthol. Finally, Citronellal forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Natural availability :
The essential oils most commonly used to collect Citronellal in its natural state are Lemongrass EO (up to 45%) or Eucalyptus citriodora (up to 80%). The addition of bisulfite salts improves the purity of the extraction.
Synthesis route :
The synthesis of citronellal is made in several possible ways. The first is from Geraniol and Nerol: a rearrangement of these molecules in the presence of a catalyst that contains oxides of copper, chromium and barium allows to obtain Citronellal as final product. Citronellol, dehydrogenated under reduced pressure, in the presence of a bichromated copper steel catalyst, also allows to obtain Citronellal. The third possibility is a Citral hydrogenation catalysed by palladium.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,41 % 0,16 % 0,026 % 0,49 % 0,33 % 0,051 % 0,1 % 0,017 % 0,82 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,077 % 0,077 % 0,017 % 1,4 % 1,4 % 2,3 % 0,017 % 0,017 % No Restriction

