Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
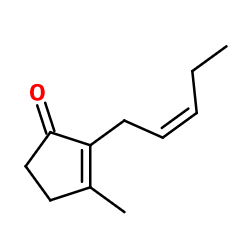
Cis-Jasmone - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 488-10-8
-
EINECS number : 207-668-4
-
FEMA number : 3196
-
Density : 0,941
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart/Base
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : 07.094
-
JECFA number : 1114
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 86°C
-
Detection Threshold : 2,9302 ng/l air
-
Molecular formula : C11H16O
-
Log P : 2,8
-
Molecular Weight : 164,25 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 121°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Dihydrojasmone has a slightlymore almond and less green smell than cis-Jasmone.
Stability :
Unstable in acidic products, except fabric conditioners, and in very alkaline detergents.
Uses in perfumery :
Cis-jasmone is used in floral notes (tuberose, jasmine, gardenia) and ambery notes. Supports Hedione® and brings depth and power to jasmine notes.
Year of discovery :
Data not available.
Isomerism :
The double bond guiding this cis-Jasmone conformation could also give rise to a trans conformation, depending on the synthesis conditions. The resulting smell would be much more earthy and close to mushroom.
Synthesis precursor :
Cis-jasmone is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Cis-jasmone is found in Grandiflorum Jasmine Absolute (and other origins), Daffodil Absolute and Champaca Absolute. It can be extracted in its natural state from Grandiflorum Jasmine Absolute.
Synthesis route :
A synthetic route of cis-Jasmone consists of a nucleophilic substitution reaction between 3-methyl-2-cyclopenten-1-one and cis-2-pentenyl chloride, in the presence of sodium hydroxide.
Regulations & IFRA
This ingredient is not restricted

