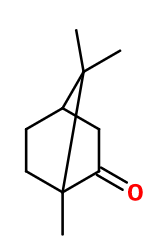
Photo credits: ScenTree SAS
General Presentation
-
CAS N° : : 76-22-2
-
EINECS number : 200-945-0
-
FEMA number : 4513
-
Density : 0,992
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : White solid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 204°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C10H16O
-
Log P : 2,41
-
Molecular Weight : 152,23 g/mol
-
Fusion Point : 176°C
-
Flash Point : 64°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Camphor is a 'trigeminal' raw material. This means that it involves the trigeminal nerve, responsible for a motor and sensitive function for humans. In the case of Camphor, smelling it gives the nose a sensation of cold.
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Camphor is used for a fresh and terpenic effect in all types of compositions.
Year of discovery :
1904
Isomerism :
There are two enantiomers of Camphor, both very present in nature. The most common is Camphor (+), dextrorotatory. It is very often this one that is used in perfumery. Citral and Isocyclocitral® are constitutional isomers of Camphor. Their smell is however very different: one is citraly, the other is green.
Synthesis precursor :
Camphor is an intermediate in the synthesis of Camphene and Borneol, among others.
Natural availability :
Camphor is found in many plants such as basil (camphorated chemotype), camphor sage and Cardamom EO among others. The Camphor tree HE (same species as HoWood EO) remains its main source in its natural state.
Synthesis route :
Camphor is produced by a fractional distillation of Camphor Leaf EO. Its synthesis is made by dehydration of isoborneol, which has been synthesized from Isobornyl acetate. This dehydrogenation is done thanks to a copper catalysis.
Regulations & IFRA
This ingredient is not restricted

