Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Calone - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 28940-11-6
-
EINECS number : 249-320-4
-
FEMA number : Donnée indisponible.
-
Density : 1,1
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€
-
Appearance : White solid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point :
-
Detection Threshold : 0,031 ng/l air
-
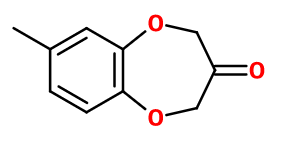
Molecular formula : C10H10O3
-
Log P : 1,95
-
Molecular Weight : 178,18 g/mol
-
Fusion Point : 39°C
-
Flash Point : 94°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
The olfactory perception threshold of Calone® is equivalent to 31 picograms / litre, that is to say a grain of salt in an Olympic pool. However, it is less powerful than Azurone®, another molecule with a marine smell.
Calone® was the initiator of the aquatic scents trend during the 90's, embodying a trend of perfumes far from the skin and closer to nature.
Its production is around 30 tons / year.
Stability :
Tends to get coloured under heat. Its smell can also change through an alcoholic maceration time.
Uses in perfumery :
Calone® is used in fresh, floral, white flower, spring and marine notes.
Year of discovery :
Discovered in 1966 by Pfizer company. Patent N°3,517,031 (US) published on Aug. 15, 1966 by Beeredoo.J, Old Lyme, Cameron.P, Stephens.C for Pfizer&Co.,Inc. Calone® brand has been published and protected by Firmenich SA since 28/08/1980
Isomerism :
Calone® does not have any isomer used in perfumery.
Synthesis precursor :
Calone® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Calone® is not available in its natural state.
Synthesis route :
Calone® is synthesized in several stages. The first one is an esterification of 4-methyl pyrocatechol with two equivalents of alkyl 2-bromoacetate. The resulting molecule undergoes a Diekmann condensation followed by an acid hydrolysis and a decarboxylation to obtain the final product.
Regulations & IFRA
This ingredient is not restricted

