Bacdanol®
Bangalol® ; Dartanol® ; Sandranol® ; Sanjinol® ; (E)-2-ethyl-4-(2,2,3-trimethyl-1-cyclopent-3-enyl)but-2-en-1-ol ; Bacdanix ; Bagdenol ; Dartanol ; Ethyl trimethyl cyclopentene butenol ; 2-ethyl-4-(2,2,2-trimethyl-1-cyclopent-3-enyl)but-2-en-1-ol ; Finalol ; Landacanol ; Levosandol ; Pracdonal ; Radjanol ; Sadacanol ; Sandal fleur ; Sandalrome ; Sandenol ; Sanderol ; Sandolene; Sandalmysore Core
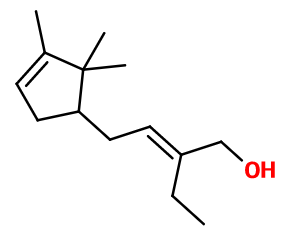
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Bacdanol - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 28219-61-6
-
EINECS number : 248-908-8
-
FEMA number : Donnée indisponible.
-
Density : 0,916
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€
-
Appearance : Colorless viscous liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 287,4°C à 760 mmHg
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C14H24O
-
Log P : 4,54
-
Molecular Weight : 208,34 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 93°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Among other sandalwood notes, Bacdanol® has a closer smell to Sandalore®, which also has a leather facet.
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Bacdanol® is used in sandalwood reconstitutions, or in combination with rosy floral notes.
Year of discovery :
Data not available.
Isomerism :
Bacdanol® has two diastereoisomers, as it has a double bond whose conformation can vary. Only the racemic mixture of the two isomers is used in perfumery.
Synthesis precursor :
Bacdanol® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Bacdanol® is not available in its natural state.
Synthesis route :
Bacdanol® is synthesized in two stages by reacting campholenaldehyde with butanal. The intermediate aldehyde that is obtained is then partially hydrogenated to turn it into alcohol.
Regulations & IFRA
This ingredient is not restricted

