Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Salicylate d'Amyle - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 2050-08-0
-
EINECS number : 218-080-2
-
FEMA number : Donnée indisponible.
-
Density : 1,054
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart/Base
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.762
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 282°C
-
Detection Threshold : Donnée indisponible.
-
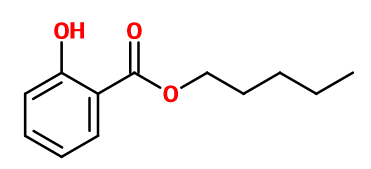
Molecular formula : C12H16O3
-
Log P : >4,4
-
Molecular Weight : 208,26 g/mol
-
Fusion Point : -12°C
-
Flash Point : 126°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Isoamyl Salicylate has a more metallic and fruity aspect, while Amyl Salicylate has a pronounced jasmine note.
It is one of the most powerful salicylates.
Stability :
May form Salicylic acid through time.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time.
Unstable in acidic products, except antiperspirants, and in bleach.
Uses in perfumery :
Amyl Salicylate is used in white flowers, carnation, fougere, ambery and vanillic notes.
Year of discovery :
Initially marketed under the name Trefol, Amyl Salicylate was first used in the perfume Trèfle Incarnat - L.T. Pivert (1898) First synthesized in 1854 by reaction of salycile chloride with amyl alcohol by Drion et al.
Isomerism :
PhenoxyEthyl IsoButyrate is a constitutional isomer of Amyl Salicylate. It smell is closer to rose than jasmine, and is not solar.
Synthesis precursor :
Amyl Salicylate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Amyl Salicylate is present in trace amounts in Osmanthus Absolute. It can therefore be extracted, but it is mostly the synthetic compound that is used in perfumery.
Synthesis route :
Like other Salicylates, Amyl Salicylate is synthesized by an esterification reaction between salicylic acid and amyl alcohol (or pentenol). This reaction is catalysed by the presence of a strong concentrated acid such as sulfuric acid in the reaction medium.
Regulations & IFRA
This ingredient is not restricted

