Alpha-ionone
α-Irisone® ; (E)-4-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-2-one ; Alphaline 70 ; 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one ; Trans-alpha-cyclocitrylidene acetone ; Ionanthem ; (3E)-4-(2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-one
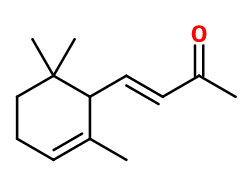
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Ionone Alpha - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 127-41-3
-
EINECS number : 204-841-6
-
FEMA number : 2594
-
Density : 0,927
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart/Base
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 07.007
-
JECFA number : 388
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 261°C
-
Detection Threshold : 0,6 à 10 ppb (0,000001%)
-
Molecular formula : C13H20O
-
Log P : 3,9
-
Molecular Weight : 192,3 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 94°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Alpha-Ionone is more floral-violet and sweeter than beta-Ionone, which is woodier. Gamma-Ionone is quite woody and resinous.
The first use of Alpha-Ionone in perfumery was in 1893, in Vera Violetta, by Roger et Gallet.
Stability :
Unstable in very acidic, except fabric conditioners, and very alkaline products
Uses in perfumery :
Alpha-Ionone is used in raspberry and floral notes of violet, orris root, rose, or carnation, for a floral-fruity and powdery facet.
Year of discovery :
This discovery was made in 1896 starting from Irones, isolated from orris butter. Ionones were discovered randomly, when a laboratory assistant added sulfuric acid to dishwash a glass containing a wrong intermediary to irone synthesis.
Isomerism :
Alpha-Ionone is obtained during the cyclization step of the pseudoionone, using sulfuric acid instead of phosphoric acid, in a catalytic amount. Gamma-Ionone is obtained by using boron trifluoride as a catalyst. Alpha-Damascone® and Beta-Damascone® are positional isomers of Ionones. Their ketone function as well as a methyl group are in different parts of the molecule. Their smell is modified: Damascone® have a smell of cooked apple instead of violet.
Synthesis precursor :
alpha-Ionone is a precursor to the synthesis of Dihydro-alpha-ionone.
Natural availability :
Alpha-Ionone is present in a few rare natural ingredients. In most cases, it is synthetic alpha-Ionone that is used in perfumery.
Synthesis route :
Nowadays, the synthesis of Ionones is made from Citral, by reaction with acetone. The category of compounds obtained is called Pseudoionone. The cyclization of Pseudoionones into Ionones is done in an acid medium. Phosphoric acid is the most preferable acid for synthesizing alpha-Ionone. The yield of this cyclization is 80% in favor of alpha-Ionone. In general, each ionone synthesis results in the formation of isomers of the desired molecule. In the case of alpha-Ionone, about 85% purity can be achieved on an industrial scale.
Regulations & IFRA
This ingredient is not restricted

