Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Allyl Amyl Glycolate - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 67634-00-8
-
EINECS number : 266-803-5
-
FEMA number : Donnée indisponible.
-
Density : 0,94
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 216°C
-
Detection Threshold : Donnée indisponible.
-
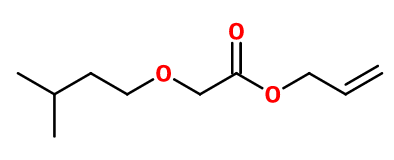
Molecular formula : C10H18O3
-
Log P : 2,72
-
Molecular Weight : 186,25 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 94°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Comparing it to Allyl Cyclohexyl Propionate, Allyl Amyl Glycolate is not as sweet as the other, and does not represent as well natural pineapple as ACHP.
Stability :
May form amyl glycolic acid through time, under the influence of heat
Uses in perfumery :
Allyl Amyl Glycolate is used in fruity, green, fougere, marine, floral and fresh notes for a slightly green fruity facet. Widely used in detergents and in any kind of perfumery, at least in trace form.
Year of discovery :
Data not available.
Isomerism :
Allyl Amyl Glycolate does not have any isomer used in perfumery.
Synthesis precursor :
Allyl Amyl Glycolate is not a precursor for the synthesis of another compound of olfactory interest.
Natural availability :
Allyl Amyl Glycolate is not available in its natural state.
Synthesis route :
Allyl Amyl Glycolate is synthesized in two stages. The first one is an esterification reaction between chloroacetic acid and isoamyl alcohol, in the presence of sodium hydroxide and a phase transfer catalyst. The second step consists in treating the intermediate product with amyl alcohol.
Regulations & IFRA
This ingredient is not restricted

