Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Aldéhyde C11 MOA - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 19009-56-4
-
EINECS number : 242-745-6
-
FEMA number : Donnée indisponible.
-
Density : 0,823
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 05.160
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
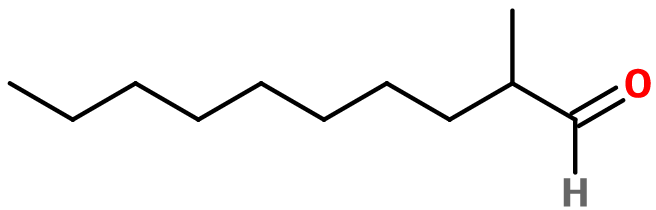
Molecular formula : C11H22O
-
Log P : Donnée indisponible.
-
Molecular Weight : 170,3 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 81°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision with Aldehyde C-12 MNA, Aldehyde C-11 MOA has one additional carbon atom. This changes it into a more fruity, a deeper and more agrestic smell.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Uses in perfumery :
Used in coriander leaves notes (Coriander Leaf EO), in sparkling liquors notes, in eaux fraiches and zesty notes, bringing an agrestic undernote.
Year of discovery :
Data not available.
Isomerism :
Aldehyde C11 MOA is a constitutional isomer of Aldehyde C11 Undecylic. It only has one ramification, while Aldehyde C11 Undecylic has a longer carbon chain. Its smell is much more fresh and zesty.
Synthesis precursor :
Aldehyde C-11 MOA forms a Schiff base by reaction with Methyl Anthranilate or Indole for example. It can also be used for condensation with another aldehyde or ketone to form the desired alcene.
Natural availability :
Aldehyde C-11 MOA is a purely synthetic aldehyde, not found in nature.
Synthesis route :
Aldehyde C-11 MOA (for Methyl Octyl Acetaldehyde) is synthesized in two different ways. 2-Decanone reacts with a Chloroacetate to give a glycidate, which can be saponified and decarboxylated to obtain the final product. The second synthesis route firstly reacts Aldehyde C-10 Decylenic with formaldehyde in the catalytic presence of an amine, and then a hydrogenation of the intermediate product allows to obtain the Aldehyde C-11 MOA.
Regulations & IFRA
This ingredient is not restricted

